Background:
The continuous production of reactive oxygen species (ROS) and oxidative stress contribute to ongoing inflammation and the pathology of sickle cell disease (SCD). ROS are known to mediate DNA damage through oxidation of nucleotide bases with guanine being the primary target. Although guanine base lesions vary according to the nature of the oxidants, 7,8-dihydro-8-oxoguanine (8-oxoG) is the most frequent oxidation product of DNA, which is repaired by 8-Oxoguanine-DNA glycosylase-1 (OGG1) via the DNA base excision repair pathway (OGG1-BER). The release of the 8-oxoG base from the genome by OGG1-BER is a prerequisite for increasing expression of pro-inflammatory cytokines and immune response. OGG1 inhibition has been shown to attenuate proinflammatory responses via the mitochondrial DNA-cGAS-STING axis in mice (Qin et al, 2020), a pathway also involved in the activation of neutrophils in SCD (Tumburu et al, 2021). Here, we evaluate the effect of OGG1 inhibition on expression of pro-inflammatory cytokines and immune response in SCD.
Methods:
Blood samples were obtained from ethnic-matched healthy controls (HbAA) and patients with SCD (HbSS) enrolled under protocols NCT03049475 and NCT00047996. CD14+ monocytes were isolated from PBMC of HbAA and HbSS subjects using EasySep™ Human Monocyte Isolation Kit (STEMCELL Technologies). 8-oxoG was measured by staining CD14+ monocytes with anti-8-oxoG antibody; OGG1 and pro-inflammatory cytokine levels were evaluated by qRT-PCR and western blotting. After differentiation, macrophages derived from CD14+ monocytes were treated with or without 10 µM SU0268 (OGG1 inhibitor) for 1 hour and then incubated with plasma from healthy controls or HbSS patients in steady or crisis status, followed by qRT-PCR quantitation of IL-1ꞵ, IL-6, TNF-α, and OGG1 mRNA levels. These measurements were repeated after OGG1 knockdown using OGG1 siRNA in macrophages derived from CD14+ monocytes.
We repeated the study on peritoneal macrophages (PMs) from Townes HbAA and HbSS mice that were collected 4 days after a 3% Brewer's thioglycolate medium intraperitoneal injection. The level of 8-oxoG was measured by staining PMs with anti-8-oxoG antibody. The mRNA and protein level of OGG1 and pro-inflammatory cytokines were evaluated by qRT-PCR and western blotting. PMs from HbAA mice were treated with 10 µM SU0268 (OGG1 inhibitor) or vehicle for 1 hour, before incubation with plasma from HbAA or HbSS mice, the mRNA levels of IL-1ꞵ, IL-6, TNF-α, and OGG1 were measured by qRT-PCR.
Results:
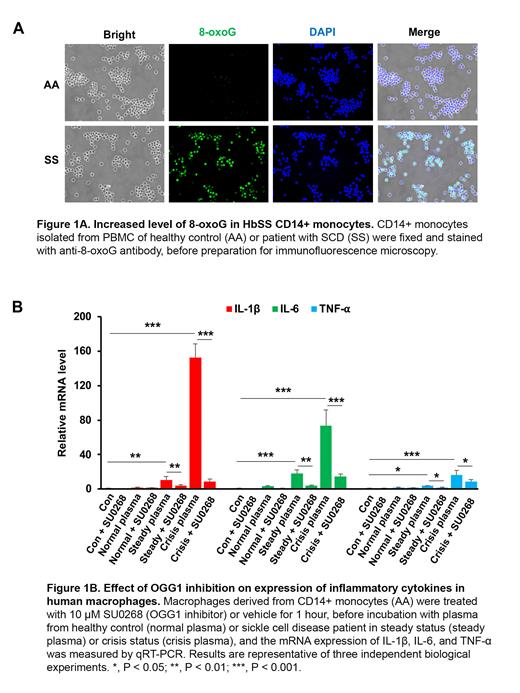
CD14+ monocytes isolated from human HbSS PBMC showed increased levels of 8-oxoG nuclear staining when compared with human HbAA CD14+ monocytes, consistent with an increased oxidative burden in SCD patients (Fig 1A). The mRNA and protein levels of OGG1 were also increased in HbSS monocytes, suggestive of a potential therapeutic target of OGG1. Consistent with previous studies, mRNA levels of pro-inflammatory cytokines IL-1ꞵ, IL-6, and TNF-α were increased in SS compared with AA monocytes. To investigate the role of OGG1 in regulating cytokine expression, we treated the macrophages derived from CD14+ monocytes with SU0268, a selective OGG1 inhibitor (Tahara et al, 2018). Macrophages treated with plasma from HbSS patients (steady and crisis status) showed increased expression of OGG1, IL-1ꞵ, IL-6, and TNF-α compared with cells treated with normal plasma, and the increase was largely blocked by SU0268 pretreatment (Fig1B). Further, siRNA knockdown of OGG1 decreased the expression of IL-1ꞵ and TNF-α, confirming the role of OGG1 in regulating pro-inflammatory cytokine expression. We then evaluated the effect of OGG1 in regulating cytokines expression in cells form SCD mice. The levels of 8-oxoG and OGG1, as well as cytokines (IL-1ꞵ, IL-6, and TNF-α), were increased in PMs obtained from HbSS mice compared with macrophages obtained from HbAA mice. Similar to the results obtained from human macrophages, murine cells treated with plasma from SS mice showed increased expression of IL-1ꞵ, IL-6, TNF-α and OGG1 compared with cells treated with plasma from HbAA mice, and pretreatment with SU0268 largely blocked their increased expression.
Conclusion:
OGG1 has a regulatory role in the expression of pro-inflammatory cytokines in SCD. OGG1 inhibition could be a potential therapeutic approach to control sickle cell inflammation.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal